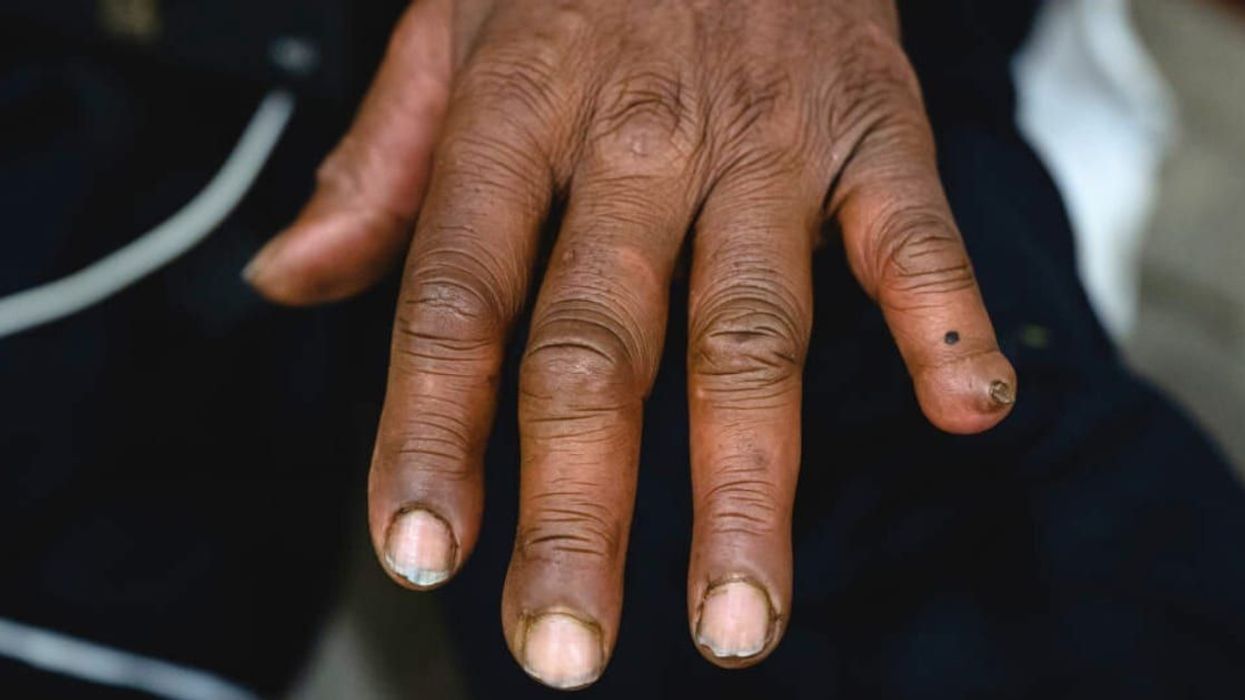
In cold conditions, people can face various health issues, including lung infections, making it crucial to keep warm. Illnesses like hypothermia and frostbite are significant risks in such temperatures. Frostbite, in particular, is a severe condition for which there is currently no medication available. At least not until now. The FDA has approved a special drug that could help cure frostbite and help prevent possible toe and finger amputation, reported Goodnews Network.
The groundbreaking drug is called Aurlumyn (iloprost) injection. It can be used to treat the injury and also provide relief to patients who have been injured by frostbite. Frostbite is caused by frozen skin. In extreme cases, it leads to tissue damage and can force people to undergo amputations. Frostbites occur mainly in the feet and toes, so it's vital for people in cold conditions to keep those body parts well-covered and warm. Aurlumyn is said to contain a special ingredient named iloprost which works to heal affected blood vessels and can also prevent blood clots in the skin.
Talking about the success of the new drug, Dr. Norman Stockbridge, director of the Division of Cardiology and Nephrology at the FDA's Center for Drug Evaluation and Research, has credited the FDA for approving the new frostbite drug. "This approval provides patients with the first-ever treatment option for severe frostbite," he told the FDA. "Having this new option provides physicians with a tool that will help prevent the life-changing amputation of one’s frostbitten fingers or toes."
However, the use of Iloprost isn't new in Canada, Nepal, and various European countries that depend on the drug. It remains to be seen how quickly pharmacists can get the drug onto the consumer market so it can start helping the general public.















 A symbol for organ donation.Image via
A symbol for organ donation.Image via  A line of people.Image via
A line of people.Image via  "You get a second chance."
"You get a second chance." 


 36 is the magic number.
36 is the magic number. According to one respondendant things "feel more in place".
According to one respondendant things "feel more in place". 
 Some plastic containers.Representational Image Source: Pexels I Photo by Nataliya Vaitkevich
Some plastic containers.Representational Image Source: Pexels I Photo by Nataliya Vaitkevich Man with a plastic container.Representative Image Source: Pexels | Kampus Production
Man with a plastic container.Representative Image Source: Pexels | Kampus Production
 Photo by
Photo by 
 Canva
Canva It's easy to let little things go undone. Canva
It's easy to let little things go undone. Canva
 Teens are waiting longer than at any point in the survey’s history. Canva
Teens are waiting longer than at any point in the survey’s history. Canva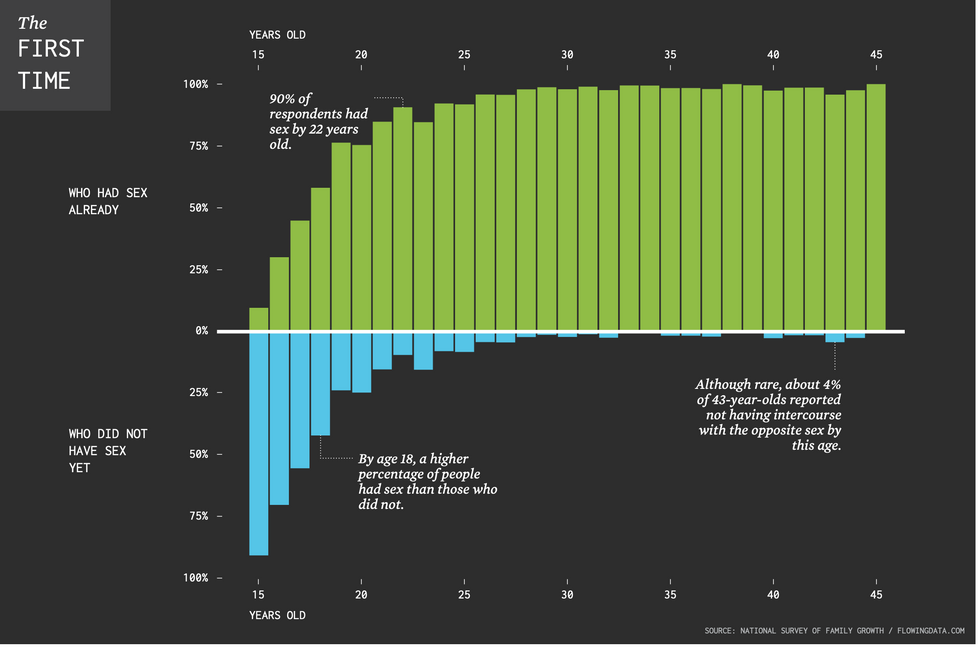 Chart on the age of a person’s first time having sex.National Survey of Family Growth/flowing data.com | Chart on the age of a person’s first time having sex.
Chart on the age of a person’s first time having sex.National Survey of Family Growth/flowing data.com | Chart on the age of a person’s first time having sex.